
 |
| LAST UPDATE 2006.03.09 |
| TOP PAGE | MEMBERS | RESEARCH | PHOTOS | NOTICES | WHAT'S NEW |
| ABOUT POLYOXOMETALATES |
|
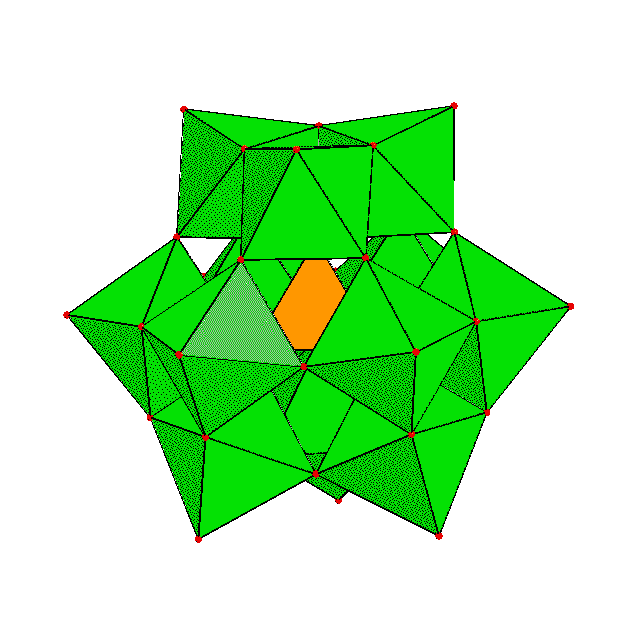
Polyoxometalates (POMs) are aggregates of oxoanions of transition metal such as V, Mo, W, Nb and Ta. These metals, which form the main framework of the POM, are called 'addenda atoms'. POMs may incorporate oxoanions of other elements such as P, Si, S, As and Ge, or other metals such as Fe, Co, Mn, Ni, Cu etc. These are called 'hetero atoms'. POMs formed by only addenda atom oxoanions are 'isopolyoxomeatlates', and those include hetero atoms are 'heteropolyoxometalates'. A number of POMs have been discovered by now, and some of them have common structural features. The most well-known structures of POMs are 'Keggin Type' with formula [XM12O40]n- and 'Dawson (or Wells-Dawson) Type' with formula [X2M18O62]n-. The most common isomers of each type are shown below. |
 |
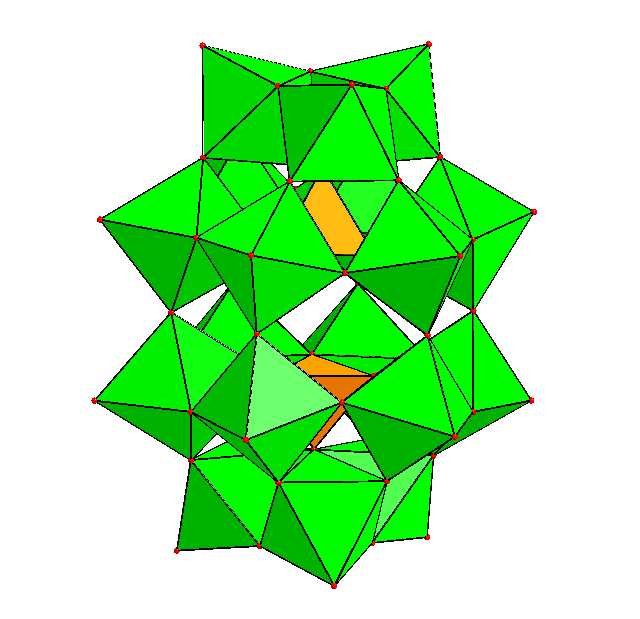 |
| a-Keggin structure | a-Dawson structure |
|
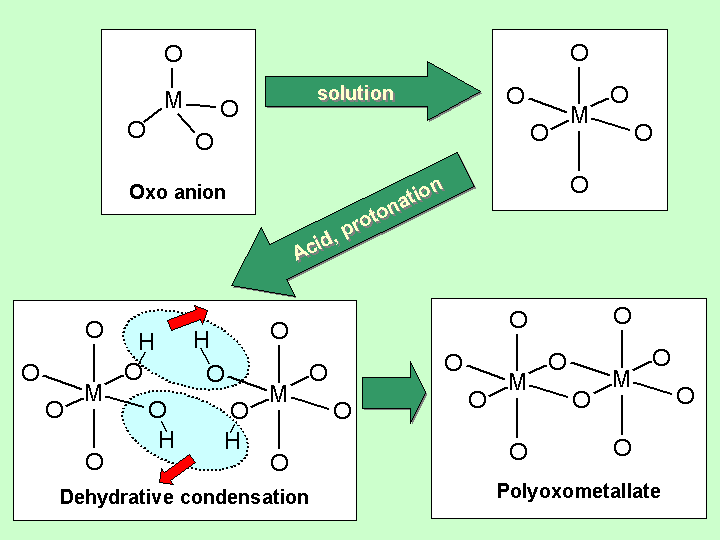
As can be seen in these figures, POMs are constructed by linkage (sharing corners, edges or faces) of polyhedra (mainly tetrahedra or octahedra, sometimes trigonal bipyramids or square pyramids) of addenda and heteroatoms. In other words, a POM is an agregate of small blocks. Diverse strctures of POMs are the results of different shapes and linkages of the blocks. The linkages are considered to be formed by dehydrative condensation. An image of the formation of a POM is like: |
 |
|
The surface of a POM is usually covered with oxygen atoms. A POM may thus be considered as an 'anionic' metal oxide or a descrete part of a metal oxide. The addenda atoms usually take their highest oxidation state, which causes peculiar redox properties of POMs. Some POMs bear rather small negative charges on rather large anions and show very strong acidity. Such features of POMs can often be modified and controled easily by changing coutercations, constructing elements etc. POMs are thus widely used as various materials, catalysts, dyes, and so on. As mentioned, POMs can be regarded as anionic oxides. By replacing oxide ligands (O2-) with peroxide ligands (O22-), peroxopolyoxometalates (PPOMs) are obtained. An addenda atom usually bears one or two peroxo group(s) on it, which results in a pentagonal bipyramidal geometry around the atom. Introduction of decahedral blocks into an aggregate leads to a novel and unique series of overall structures of PPOMs. PPOMs are expected to be active oxygen carriers in oxidation reactions, potential precursors of oxide materials, photoregistic materials, etc. However, PPOM chemistry is not well-established so far. Our group is thus working with synthesis, structure, reaction and property of PPOMs, aiming at establishing 'PPOM chemistry'. |
| USE OF ANY CONTENT IN THIS PAGE WITHOUT PERMISSION IS STRICTLY FORBIDDEN. |
| THE WEBMASTER DOES NOT TAKE ANY RESPONSIBILITY FOR ANY PROBLEM OR INCONVENIENCE CAUSED BY USING ANY CONTENT OF THIS PAGE. |
| ANY QUERY ON THIS PAGE SHOULD BE FORWARDED TO THE WEBMASTER. |